Background: Hepcidin is the master regulator of iron homeostasis. Its expression is controlled, in part, by signaling through TGF-β receptors including activin receptor-like kinase-2 (ALK2). As part of a negative feedback loop, high hepcidin levels increase expression of matriptase-2, which cleaves the ALK2 co-receptor hemojuvelin from the cell surface and suppresses ALK2 signaling. Dysregulation of the negative feedback system results in high hepcidin, which mobilizes iron from storage tissues and leads to insufficient iron for red blood cell production in the bone marrow, resulting in anemia. KER-047 is a selective ALK2 inhibitor that has the potential to normalize ALK2 signaling and the downstream sequalae that results in anemia as a result of elevated hepcidin.
Aims: The objective of this Phase 1 study was to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamic effects of single and multiple ascending dose levels of KER-047 in healthy participants.
Methods: In Part 1, two formulations of KER-047 were evaluated in single ascending oral doses ranging from 1 mg to 300 mg of a capsule formulation, and 30 mg to 450 mg of a liquid formulation or placebo. In Part 2, the liquid formulation was evaluated in multiple ascending doses (50 mg, 100 mg, 200 mg and 350 mg) of KER-047 or placebo, administered daily for up to 7 days. Endpoints included adverse events (AEs) and pharmacokinetic and pharmacodynamic parameters. Informed consent was obtained from all participants prior to enrollment in the study.
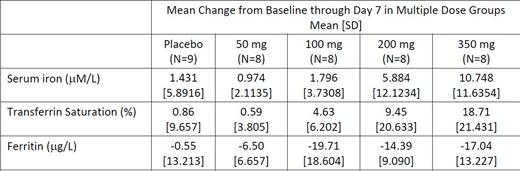
Results: Part 1 enrolled 80 participants; 60 received a single dose of KER-047 and 20 received placebo. Part 2 enrolled 41 participants; 32 received KER-047 and 9 received placebo daily for up to 7 days. There were no serious adverse events in either part of the study. In Part 1, 3 (9.4%) participants discontinued the study; none discontinued due to AEs. In Part 2, 3 (9.4%) participants administered KER-047 and 1 (11.1%) placebo discontinued the study; of these, 2 participants discontinued due to an AE (one in the 200 mg group and one on placebo). In Part 2, 1 of 8 (12.5%) administered 200 mg and 4 of 8 (50%) participants administered 350 mg discontinued study drug due to AEs. The majority of AEs observed in participants administered KER-047 were mild or moderate in severity; severe AEs were reported only at the 350 mg multiple dose in 1 of 8 (12.5%) participants. AEs reported in ≥2 of participants administered KER-047 and higher than placebo were: headache, nausea, vomiting, diarrhea, gastroenteritis, chills, pyrexia, myalgia, decreased appetite, lymphopenia, neutropenia, and liver enzyme increases. Decreases in lymphocyte and neutrophil counts were observed at 200 mg and 350 mg. Mean KER-047 AUC and Cmax increased linearly, with greater than dose-proportional increases observed across multiple doses from 50-200 mg. Half-life values ranged from approximately 10 to 15 hours. Administration of KER-047 elicited rapid, robust and sustained dose-related increases in serum iron and transferrin saturation. Increases in serum iron and transferrin saturation were observed beginning on Day 2 after single doses and were sustained after multiple doses. Decreases in ferritin were also observed. Decreases in serum hepcidin post-dose were also observed in all the multiple dose cohort in which changes were evaluated. Reticulocyte hemoglobin content increased starting on Day 3 post-dosing indicating increased iron availability in the bone marrow.
Summary: In healthy participants, administration of KER-047 elicited rapid, robust and sustained dose-related increases in serum iron and transferrin saturation that were associated with decreases in ferritin and hepcidin. The observed decrease in ferritin and hepcidin coupled with increases in reticulocyte hemoglobin content are indicative of increased iron mobilization, resulting in increased iron incorporation into hemoglobin. The tolerability profile of KER-047 in healthy participants has been characterized in this Phase 1 study. KER-047's unique pharmacologic effect on hepcidin and iron mobilization has the potential to treat anemia that results from elevated hepcidin.
Ordonez:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company; Ackea Therapeutics: Ended employment in the past 24 months. Ben:Nucleus Network: Current Employment. Barger:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company. Serino:BioBridges: Ended employment in the past 24 months; Keros Therapeutics: Current Employment. Tseng:Mitobridge: Current equity holder in private company; Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Rovaldi:Keros Therapeutics: Consultancy. Lachey:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company. Seehra:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal